The NEBExpress MBP Fusion and Purification System takes advantage of the strong Ptac promoter and the translation initiation signals of maltose binding protein (MBP) to enhance solubility and expression levels of a desired protein in E. coli.
The resulting product is an MBP fusion protein, which is then purified by affinity chromatography.
The included pMAL-c6T vector expresses the N-terminal hexahistidine tagged malE gene (lacking its secretory signal sequence and engineered for tighter binding to amylose) followed by a multiple cloning site containing a TEV protease recognition sequence and stop codons in all three frames. The pMAL-c6T vector expresses the MBP fusion in the cytoplasm.
NEBExpress MBP System - Workflow
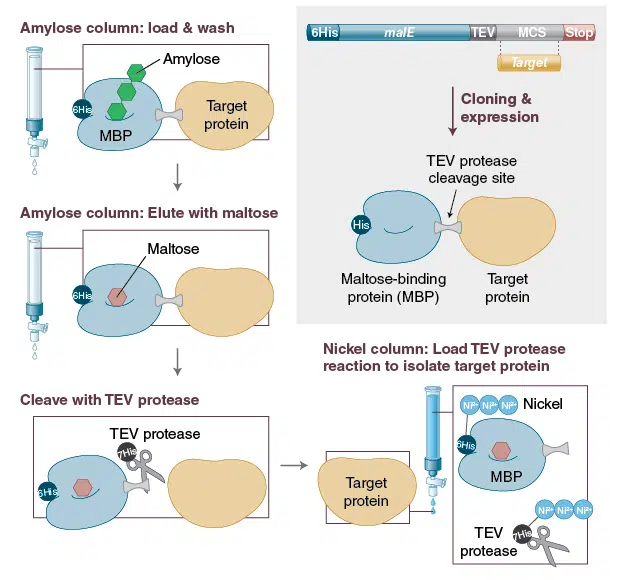
The fusion protein can be purified by a one-step purification method using an amylose matrix and MBPs affinity for maltose. Small-scale protein purification can directly be done from 200 – 500 µl culture supernatant, while large-scale purifications are possible from up to 1000 ml of bacterial culture.
Following amylose purification, the target protein can be cleaved from the MBP-tag using TEV Protease, without adding any vector-derived residues to the protein. Both the MBP-tag and TEV Protease are polyhistidine-tagged for easy removal from the reaction. The target protein yield can be up to 100 mg/l, with typical yields in the range of 10–40 mg/l.
- Reliable E. coli expression: substantial yields (up to 100 mg/L)
- Fusion to MBP has been shown to enhance the solubility of proteins expressed in E. coli
- Two-step purification: amylose elution followed by TEV Protease cleavage and Ni resin isolation results in a highly pure tag-free target protein
- Gentle elution with maltose; no detergents or harsh denaturants required
The NEBExpress MBP Fusion and Purification System contains all reagents for standard protein expression and purification:
- pMAL-c6T Vector
- Amylose Resin
- TEV Protease (incl. reaction buffer)
- Antibodies and control for Western Blot analysis
- E. coli NEB Express
Separately available:
As of: 01.01.2024
Further information can be found in our Technical Resources section or at neb.com. Information on trademarks can be found here.

