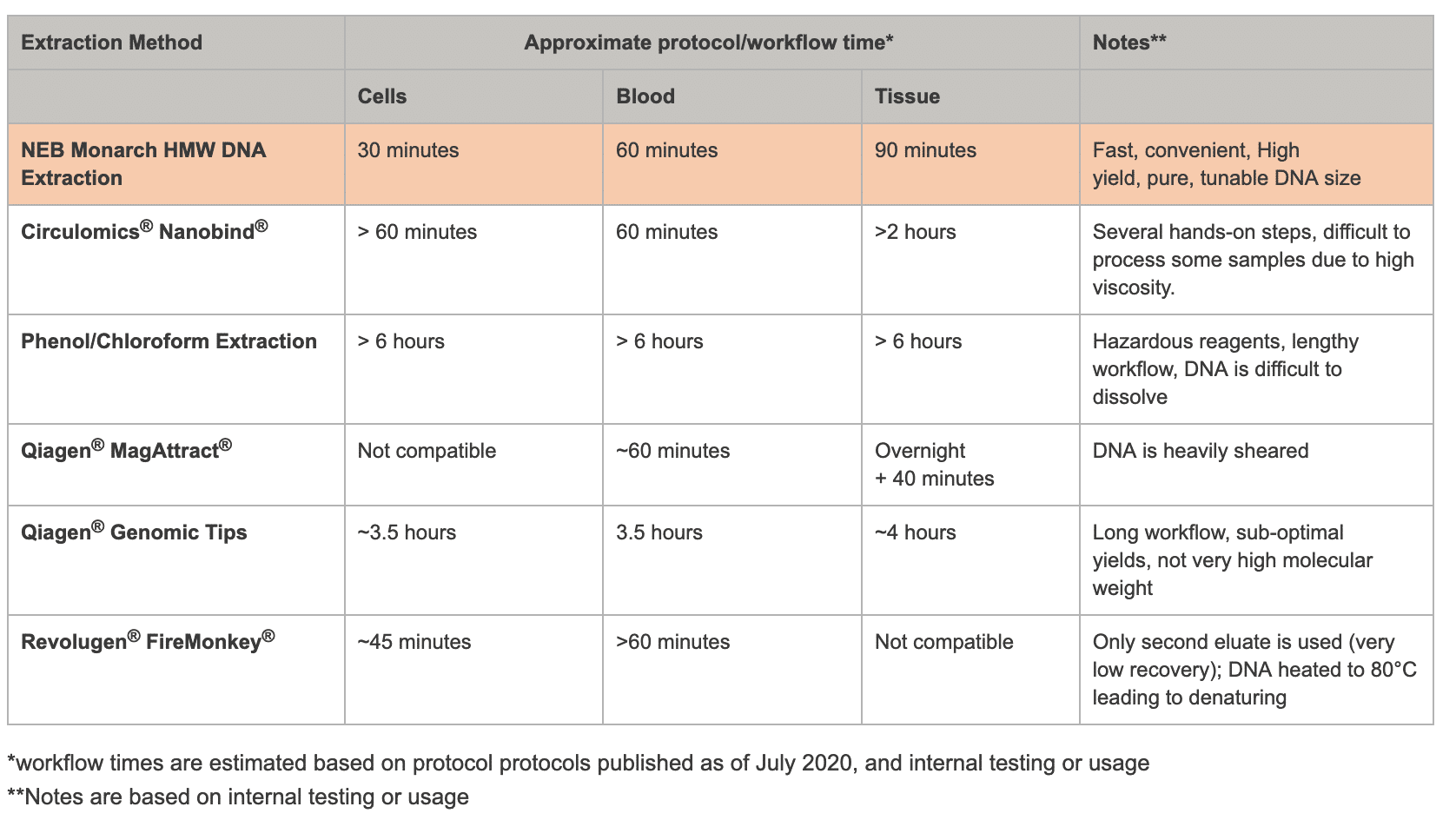
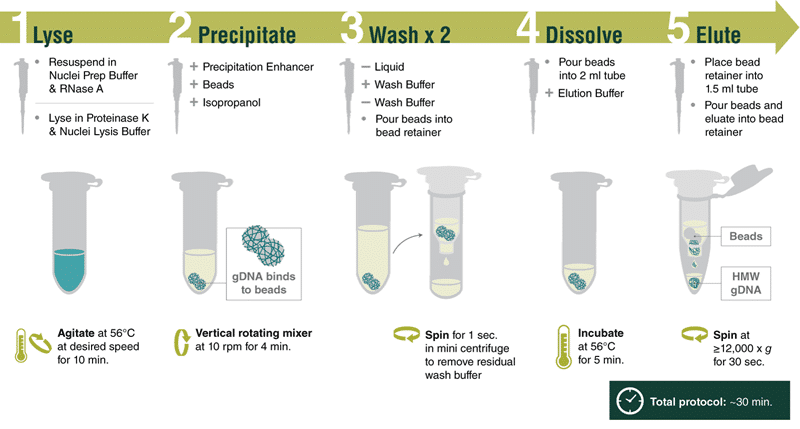
High Molecular Weight DNA Extraction
The Monarch HMW DNA Extraction Kits provide a rapid and reliable process for extracting high molecular weight DNA (HMW DNA) from biological samples. These kits utilize an optimized process that combines gentle cell lysis with a tunable fragment length generation, followed by precipitation of the extracted DNA onto the surface of large glass beads. DNA size ranges from 50-250 kb for the standard protocol and into the Mb range for certain sample types when the lowest agitation speeds are used. Purified DNA is recovered in high yield with excellent purity, including nearly complete removal of RNA, and is ready for use in downstream applications including long-read sequencing.
- Extremely fast, user-friendly protocols utilizing a novel glass-bead-based approach
- Reproducibly purify high molecular weight genomic DNA (HMW DNA) from various sample types
- Tune fragment length by varying agitation speeds during lysis; achieve DNA in the Megabase size range with low speeds
- Achieve outstanding results when compared to other commercially available solutions
- Excellent performance in long read sequencing
Extremely fast, user-friendly protocols utilizing a novel glass-bead-based approach:
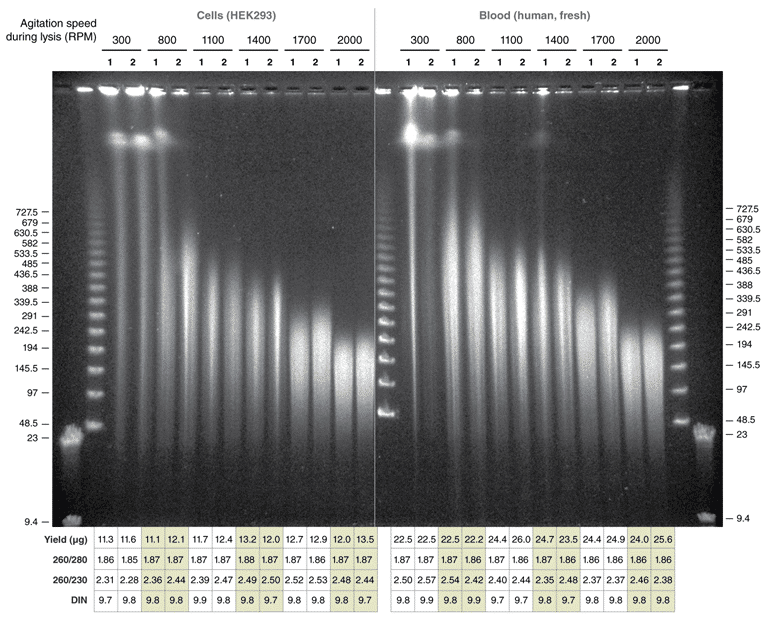
Reproducible Extraction of HMW DNA from Cells and Blood with the Monarch HMW DNA Extraction Kit:
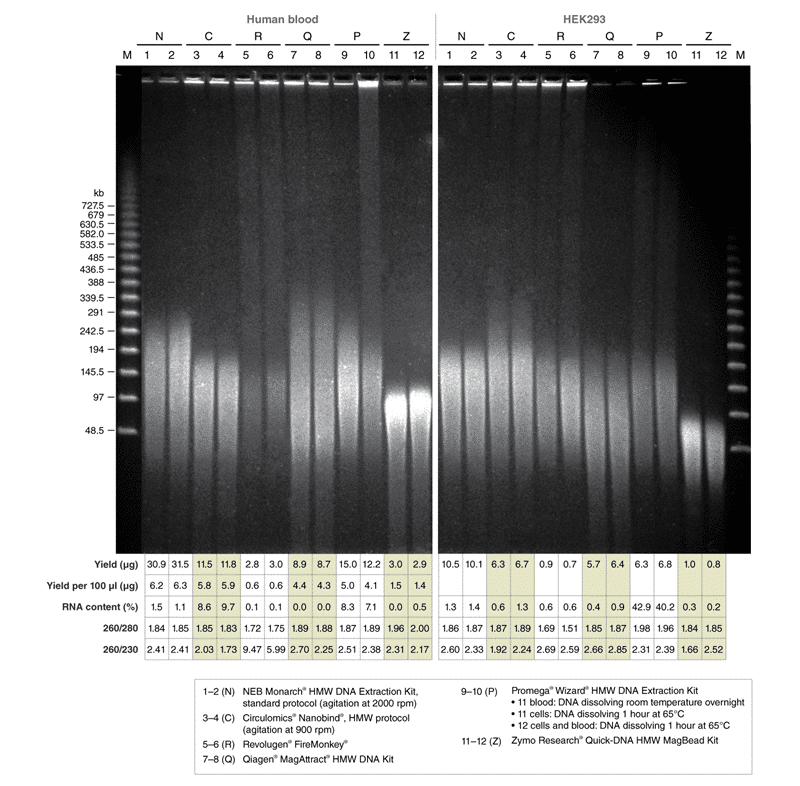
DNA extracted with Monarch HMW DNA Extraction Kit for Cells & Blood. 1 x 106 fresh HEK293 cells and 500 μl fresh human blood were used as inputs and for preps performed according to the kit instructions using the agitation speed indicated above the gel lanes. 500 ng of DNA from the replicates was resolved by PFGE (1% agarose gel, 6 V/cm, 13°C for 20 hours, switch times ramped from 0.5–94 seconds on a BioRad® CHEF-DR® III System). Yield and purity ratios of the individual preps are shown in the accompanying tables. Lambda PFG Ladder (NEB #N0341) was used as molecular weight standard.
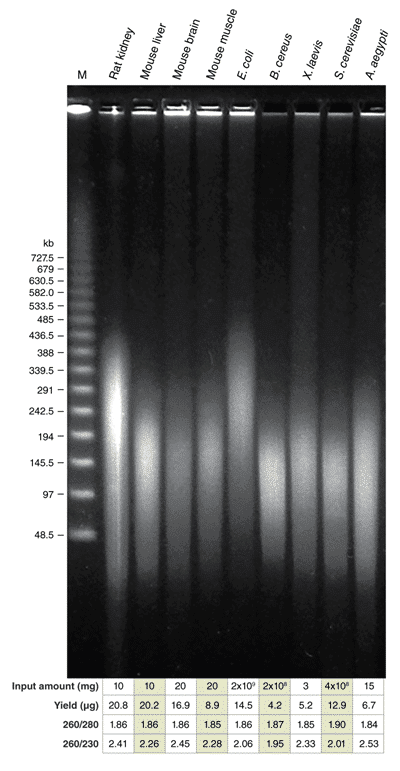
The Monarch HMW DNA Extraction Kit for Tissue efficiently purifies high-quality, HMW DNA from a variety of sample types :
HMW genomic DNA extracted with the Monarch HMW DNA Extraction Kit for Tissue. 10 mg frozen rat kidney, 10 mg fresh mouse liver, 20 mg frozen mouse brain, 20 mg fresh mouse muscle, 2 x 109 frozen E. coli cells, 2 x 108 frozen B. cereus cells, 4 mg fresh X. laevis, 3.8 x 108 fresh S. cerevisiae cells and 15 mg frozen A. aegypti were used as inputs for preps. Preps were performed according to the kit instructions with sample agitation at 2000 rpm. A modified workflow was used to process S. cerevisiae preps. 500 ng of DNA from each sample prep was resolved by PFGE (1% agarose gel, 6 V/cm, 13°C for 20 hours, switch times ramped from 0.5-94 seconds on a BioRad CHEF-DR III System). Yield and purity ratios of the individual preps are shown in the accompanying tables. Lambda PFG Ladder (NEB #N0341) was used as a molecular weight standard.
HMW was isolated from 1×106 HEK293 cells and fresh human blood with the kits indicated in the figure legend. Blood input volumes were used as specified in manufacturers’ protocols (N: 500 μl, C: 200 μl, R: 500 μl, Q: 200 μl, P: 300 μl, Z: 200 μl).
Monarch samples (lanes 1-2) were purified at maximum agitation speed during lysis (2000 rpm). Variation in fragment length of cellular DNA using the standard protocols for Monarch and Circulomics (lanes 1-2 and 3-4, respectively) results from agitation speeds during lysis (Monarch: 2000 rpm, Circulomics: 900 rpm). All other data presented are duplicate samples from each different kit and the standard protocols dedicated to blood or cells were followed. Qiagen does not provide a protocol for cultured cells; a modified version of the blood protocol was followed. Samples were eluted in 100 μl, except for Zymo which was eluted in 50 μl according to their recommendations.
Yield and purity of the standard samples were analyzed on Trinean Dropsense 16 spectrophotometer (now Unchained Labs Lunatic. Reported blood sample yields were normalized per 100 μl. RNA content was determined by HPLC analysis of nucleoside content after digestion of 1 μg of eluted DNA with the Nucleoside Digestion Mix (NEB #M0649) . The optional RNase treatment was performed with the Zymo prep.
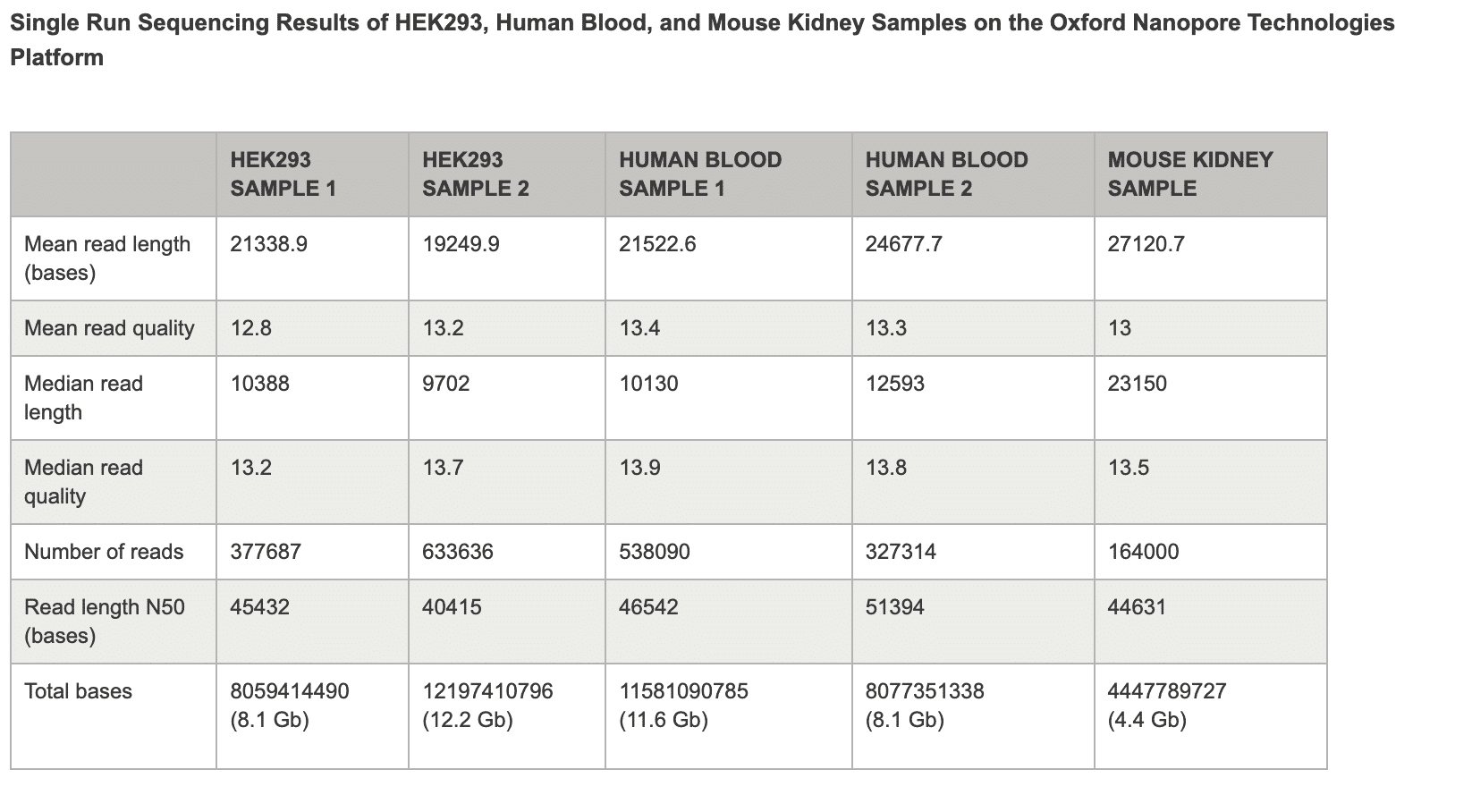
DNA used for the sequencing libraries was extracted by following the standard protocols for cultured cells and fresh mammalian blood samples, without further size selection. Mouse kidney DNA samples were extracted from fresh samples using a rotor-stator homogenizer for sample homogenization. Libraries were generated using the LSK109 ligation sequencing kit and loaded on a FLO-MIN106D flow cell. Sequencing was performed on a GridION® Mk1 for up to 48 hours, or shorter if no more data was generated by the flow cell. No additional treatment of the flow cell (e.g., flushing) was employed. In the samples that resulted in > 10 Gb of data, 800–1,000 ng of DNA library was loaded onto the flow cell for optimal sequencing performance and effective pore usage. Read lengths are indicated in bases unless otherwise noted.
Available Monarch HMW DNA Extraction Kits and companion products:
As of: 01.01.2024
Further information can be found in our Technical Resources section or at neb.com. Information on trademarks can be found here.